The US Food and Drug Administration (FDA) plays an important role in ensuring the safety of the drugs we consume. Every year, the FDA conducts drug recalls to remove pharmaceuticals from the market due to safety concerns. But how many drugs have been recalled by the FDA in recent years? In this article, we’ll explore the number of drugs that have been recalled by the FDA, as well as the reasons behind the recalls.
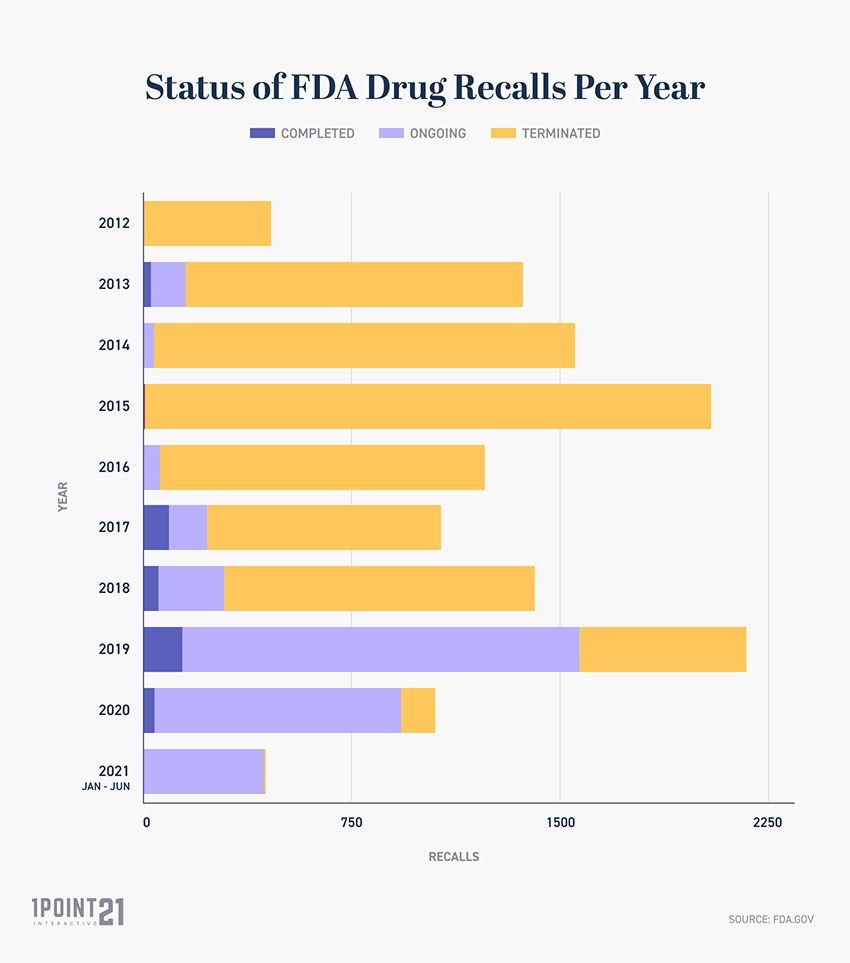
According to the U.S. Food and Drug Administration (FDA), there have been over 3,000 drug recalls since 2000. Most of the recalls are Class II, which means that the recalled drugs may cause temporary or medically reversible adverse health consequences.
Contents
What Are Drug Recalls by the FDA?
Drug recalls by the FDA are when the Food and Drug Administration (FDA) has identified a safety issue with a drug and has issued a recall to remove the product from the market. The FDA may issue a recall due to safety concerns, such as the presence of contaminants or a lack of effectiveness of the drug. Drug recalls can also be voluntary or mandatory, depending on the severity of the risk posed by the product.
The FDA is responsible for monitoring the safety of drugs, including the approval of new drugs and the monitoring of existing drugs for safety and effectiveness. The FDA can also issue warnings and recalls if a drug is found to be unsafe or ineffective. The FDA’s goal is to ensure that drugs are safe and effective for use by consumers.
The FDA reviews drug safety data and may issue a recall if a safety concern is identified. The FDA has the authority to order a recall of a drug if the risk posed by the drug is significant. The FDA also has the authority to suspend the sale of a drug if the risk posed by the drug is too great.
What Types of Drugs Have Been Recalled?
The types of drugs that have been recalled by the FDA have ranged from over-the-counter medications to prescription drugs. Over-the-counter medications include pain relievers, cold and cough medications, and dietary supplements. Prescription drugs include antibiotics, antivirals, hormones, and other medications.
In some cases, the FDA has identified potential safety issues and recalled the drugs before they were available to consumers. In other cases, the FDA has identified potential safety issues after the drug was already on the market and issued a recall to remove the product from the market.
The FDA can also issue recalls of drugs that have been approved for use but have been found to be ineffective or to cause serious side effects. In some cases, the FDA may also recall drugs that have been approved but have been found to be ineffective or to cause serious side effects in a small number of users.
How Many Drugs Have Been Recalled by the FDA?
Since 2007, the FDA has recalled a total of 6,867 drugs. The majority of these recalls were due to safety concerns, such as the presence of contaminants or a lack of effectiveness of the drug. The most common type of recalled drug was an over-the-counter medication, such as a pain reliever, cold and cough medication, or dietary supplement.
The FDA has also issued recalls for a variety of prescription drugs. For example, in 2018, the FDA issued a recall for a generic version of the popular cholesterol-lowering drug Lipitor due to a potential contamination with glass particles. The FDA has also issued recalls for other drugs, such as antibiotics, hormones, and other medications.
How Are Drug Recalls Handled by the FDA?
When the FDA issues a recall for a drug, the manufacturer or distributor of the drug is responsible for notifying the public of the recall and removing the product from the market. The manufacturer or distributor must also provide the FDA with a plan for how the recall will be handled, including how the product will be returned to the manufacturer and how the public will be informed of the recall.
The FDA also has the authority to suspend the sale of a drug if the risk posed by the drug is too great. The FDA may also issue warnings and recalls if a drug is found to be unsafe or ineffective.
What Are the Consequences for a Drug Recall?
When a drug is recalled by the FDA, the manufacturer or distributor of the drug is responsible for notifying the public of the recall and removing the product from the market. The manufacturer or distributor may also be subject to fines or other penalties if they fail to comply with the FDA’s requirements. In some cases, the FDA may also require the manufacturer or distributor to provide refunds to consumers who purchased the recalled product.
Frequently Asked Questions
What is the FDA?
The Food and Drug Administration (FDA) is an agency within the U.S. Department of Health and Human Services. It is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices. It also regulates the manufacture, labeling, and distribution of food in the United States.
What is a drug recall?
A drug recall is a voluntary action taken by a manufacturer or the FDA to remove a drug from the market. Recalls are initiated when the FDA or a manufacturer determines that a drug may be contaminated, mislabeled, ineffective, or potentially harmful.
How many drugs have been recalled by the FDA?
Since 2003, the FDA has recalled over 2,000 drugs, including both prescription and over-the-counter medications. Some of the most commonly recalled drugs are those used to treat high blood pressure, cholesterol, and diabetes.
What happens when a drug is recalled?
When a drug is recalled, the FDA issues a public warning to alert consumers and healthcare professionals about the dangers associated with the product. The FDA also works with manufacturers to ensure that the recalled product is removed from the market.
Are recalled drugs safe to use?
No, recalled drugs are not safe to use. The FDA recommends that consumers and healthcare professionals stop using the recalled product immediately and dispose of it properly.
What should I do if I have a recalled drug?
If you have a recalled drug, you should stop using it immediately and contact your doctor or pharmacist for advice. Your doctor or pharmacist can help you find an alternative treatment option. You should also contact the manufacturer directly to return the product and receive a refund.
Your drug has been recalled – what do you do?
The FDA has recalled countless drugs due to safety concerns, ranging from minor to severe. It is important for consumers to be aware of any recalls, so they can take the necessary precautions to protect their health. If a drug has been recalled, it is best to contact your doctor or pharmacist to discuss alternative treatment options. It is essential to stay informed about the safety of drugs, as this could be the difference between life and death.
