The Food and Drug Administration (FDA) is responsible for ensuring the safety of drugs, foods, and medical devices. The FDA makes sure that drugs are effective and meet certain standards. But does the National Institutes of Health (NIH) also approve drugs for the FDA?
The answer is yes, the NIH plays an important role in the approval of drugs for the FDA. The NIH works closely with the FDA to ensure that drugs meet certain safety and efficacy standards before they are approved for use. The NIH also conducts research to determine the potential risks and benefits of drugs and provides input to the FDA on the safety and effectiveness of drugs. This ensures that the drugs approved by the FDA are safe and effective for use by the public.
No, the National Institutes of Health (NIH) does not approve drugs for the Food and Drug Administration (FDA). The FDA is responsible for the safety and efficacy of drugs and medical devices, while the NIH is responsible for conducting and supporting biomedical research.
The FDA is part of the U.S. Department of Health and Human Services and is responsible for reviewing and approving new drugs and medical devices. The FDA reviews data from clinical trials and other sources to determine if a drug or device is safe and effective for its intended use. The FDA also monitors products for safety and efficacy after they are approved and available for use in the US.
The NIH is part of the U.S. Department of Health and Human Services and is responsible for conducting and supporting biomedical research. It is the largest public funder of biomedical research in the world and is responsible for advancing knowledge about human health and disease.
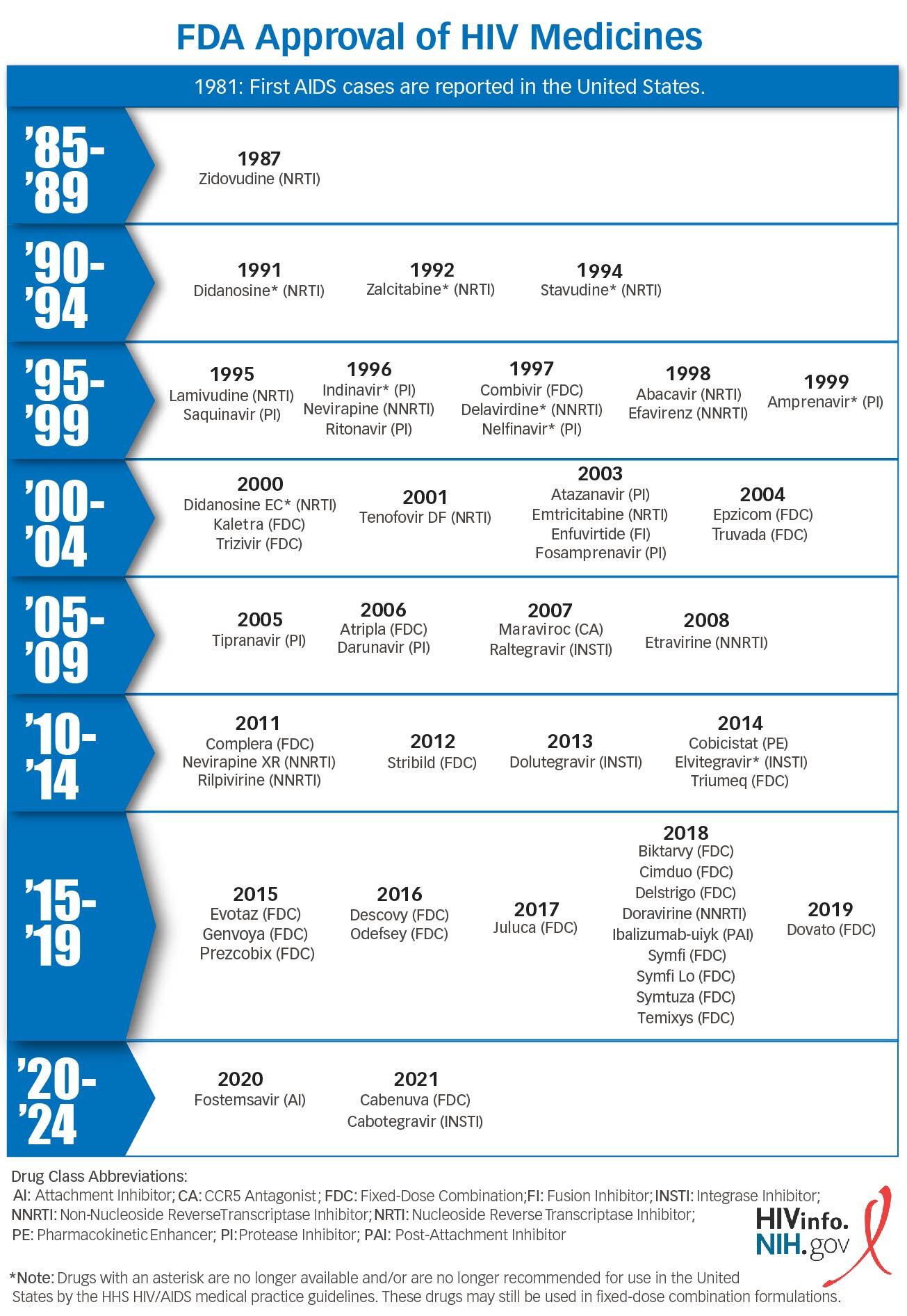
Contents
Does the NIH Approve Drugs for the FDA?
The National Institutes of Health (NIH) is a federal agency responsible for conducting medical research and providing funding for medical research. The NIH does not have the authority to approve drugs for the Food and Drug Administration (FDA). The FDA is the agency responsible for ensuring that drugs are safe and effective before they can be sold in the United States.
The Role of the NIH in Drug Development
The NIH plays an important role in drug development. The NIH provides funding for research into new drugs and treatments, and it also funds clinical trials to test the safety and effectiveness of new drugs. The NIH also provides advice and guidance to drug companies on safety and other issues related to drug development. The NIH does not, however, have the authority to approve drugs for use in the United States.
The Role of the FDA in Drug Approval
The FDA is responsible for approving drugs for use in the United States. The FDA reviews the safety and effectiveness of drugs and will only approve a drug if it believes the drug is safe and effective for its intended use. The FDA evaluates the potential risks of a drug and also assesses the benefits of the drug before deciding whether to approve it. The FDA also has the authority to recall drugs from the market if they are found to be unsafe or ineffective.
Frequently Asked Questions
The National Institutes of Health (NIH) is the largest biomedical research agency in the world and a part of the U.S. Department of Health and Human Services. The NIH works to develop treatments, cures, and preventions for diseases and conditions. The U.S. Food and Drug Administration (FDA) is responsible for protecting public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices.
Does the NIH approve drugs for the FDA?
No, the NIH does not approve drugs for the FDA. The FDA is responsible for approving all drugs that are marketed in the United States. The FDA reviews applications from drug sponsors and makes decisions about whether to approve, deny, or place a drug on hold. The NIH may play a role in the drug approval process by providing research and scientific advice to the FDA.
The NIH may also provide funding for research and clinical trials that are needed to support a drug’s approval. The NIH may also provide technical assistance to drug sponsors in conducting clinical trials. However, the NIH does not have the authority to approve or deny drugs that are submitted to the FDA for approval.
In conclusion, it is clear that the NIH plays a critical role in the approval of drugs for the FDA. The NIH is responsible for evaluating the safety, efficacy, and quality of drugs before they can be approved by the FDA. The NIH works closely with the FDA to ensure that all drugs are safe and effective for the public.
The NIH and FDA have a strong working relationship and work together to ensure that only safe and effective drugs make it to the public. The NIH and FDA have strict guidelines when it comes to drug approval, and they take the safety of the public very seriously. Ultimately, the NIH and FDA are working together to ensure that only safe and effective drugs are approved for the public.
